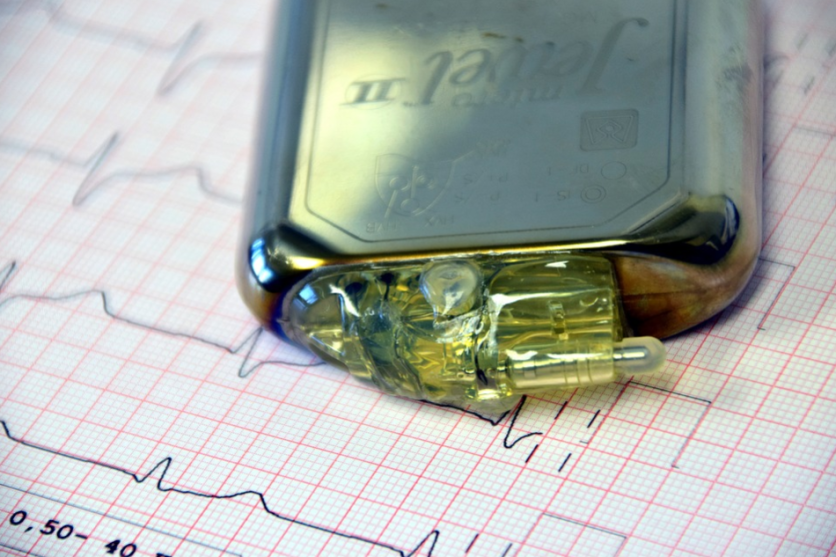
The American Heart Association (AHA) says the device equipped with Apple's MagSafe technology can cause interference when they are placed over the skin or if they are in very close proximity to cardiac devices like pacemakers.
AHA researchers found that select devices from all of the three major cardiac device companies: Abbott, Medtronic, and Boston Scientific, were found to have magnetic susceptibility.
AHA Concerns About MagSafe
The researchers wrote in the paper that was published by AppleInsider that their study demonstrates that magnet reversion mode may be triggered when the iPhone 12 Pro Max is placed directly on the skin over an implantable cardiac device and thus has the potential to inhibit lifesaving therapies.
The researchers tested the effect of Apple's MagSafe technology on cardiac devices by placing the iPhone very close to 11 pacemakers and defibrillators. Some of the devices were implanted in patients, and others were new and unboxed.
Placing the iPhone on top of the skin or very close to devices resulted in clinically identifiable magnet interference in all three new devices that were tested and in eight out of 11 unboxed devices.
The iPhone was able to trigger magnetic reversion mode on one unboxed device at a distance of up to 1.5cm.
Apple stated in an advisory that the iPhone 12 does not pose a greater risk for magnet interference when compared to the older generation iPhones.
The paper, which was also published in the Journal of the American Heart Association, noted that the study suggests otherwise as magnet response was demonstrated in 3/3 cases in new devices.
In comparison to the older generation iPhone 6, a study performed by Lacour found no cases of magnet response in a sample size of 148 patients.
The potential interference could happen when someone places a MagSafe device in a coat or shirt pocket over their chest, which the researchers say can eventually lead to asynchronous pacing or disabling of antitachycardic therapies.
A strong external magnet can also cause issues with a lot of cardiac implantable electronic devices or CIED, as the study notes, so the issue is not exclusive to MagSafe technology.
AHA Pushes for More Research
The researchers say that their findings highlight the importance of public awareness regarding interaction between CIEDs and a recently released smartphone model with magnetic charging capability.
Even though the Food and Drug Administration or FDA website states that cellphones do not pose any health risk for patients with cardiac devices, they do acknowledge that certain precautions are advisable.
In January, Apple revised its MagSafe technology support document. It recommends that iPhone 12 users should keep the device at least six inches away from medical implants.
The authors of the paper also suggested patients should consult with a heart rhythm specialist regarding recommendations specific to their smartphone and CIED.
AppleInsider noted that the study's findings align with one from the Heart Rhythm Journal, which pointed out that the iPhone 12 can affect CIEDs when it is placed on or very close to the implant. The AHA paper also recommends a wider study of the MagSafe issue.
This article is owned by Tech Times
Written by Sophie Webster




