The rapidly growing trend of medical spas and hydration clinics in the United States has caught the attention of federal health officials due to increasing reports of health complications.
These facilities, offering an array of services from vitamin shots to hydrating intravenous infusions, have become a $15 billion industry. Healio reported that there were 7,430 facilities in the US in 2021, each earned $1,722,551 in revenue. The number of medical spas grew In 2022, with 8,841 and each earned $1,982,896 on average.
According to BeautyMatter, social media is significantly influencing perceptions of aesthetic procedures. As consumers increasingly research health matters online before consulting a doctor (nearly 60%), the cosmetic industry sees extensive digital marketing potential.
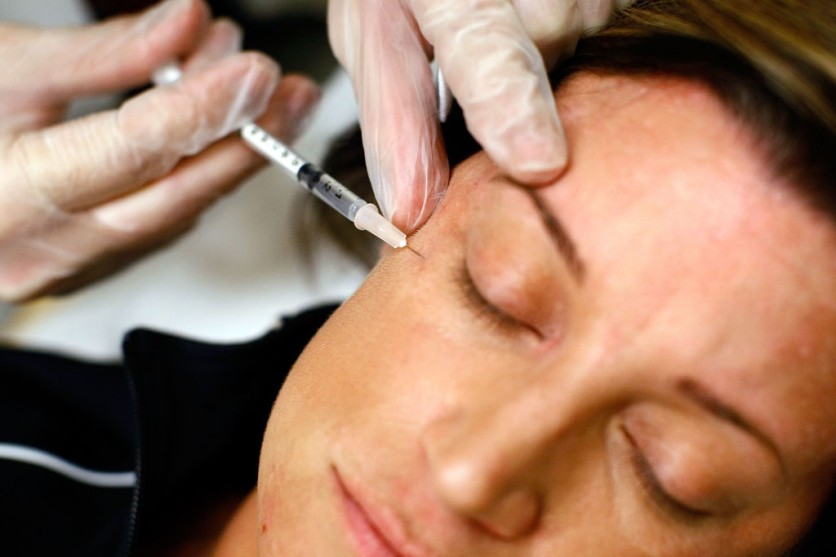
Questionable Wellness Treatment
However, concerns have been raised about the risks associated with unproven treatments and the lack of oversight leading to potential health hazards. Dr. Richina Bicette-McCain, an emergency medicine physician at Baylor College of Medicine, cautions against the unverified medical benefits promised by so-called wellness treatments. She emphasizes that the purported advantages are not medically proven, urging consumers to exercise caution.
The Food and Drug Administration (FDA) recently issued warnings following reports of consumers developing severe infections and skin deformities after receiving unauthorized shots designed to dissolve fat.
Sold online under various names, such as Aqualyx, Lipodissolve, Lipo Lab, and Kabelline, these unapproved products have raised significant health concerns. The FDA highlighted cases where consumers received injections from personnel who might not have been properly licensed, emphasizing the potential dangers associated with such practices.
The only FDA-approved product for breaking down fat under the skin is Kybella, typically administered underneath the chin to address a double chin. The use of unauthorized alternatives raises serious health risks and concerns about the qualifications of personnel administering these treatments.
Authorities Point Out Serious Risks
Dr. Bicette-McCain has observed a growing number of individuals experiencing adverse reactions after visiting medical spas or hydration clinics. Infections, particularly at the site of intravenous (IV) placement, have become one of the most common complications. Additionally, patients have reported burns from laser treatments and sought assistance following poorly administered Botox injections, per NBC News.
The FDA's warnings extend beyond individual cases, pointing to issues with med spas and IV clinics, including mobile IV infusion services, and mixing products inappropriately without adhering to proper sterilization procedures. Contaminated or poor-quality compounded drug products can lead to severe patient illnesses, including death.
A significant challenge in addressing these concerns lies in the lack of federal health regulations or standard operating procedures for medical spas. Alex Thiersch, the chief executive of the American Med Spa Association, acknowledges the absence of consistent oversight, with each state responsible for regulating these facilities. Unfortunately, enforcement is often lacking due to resource constraints in certain states.
To bridge this regulatory gap, the American Med Spa Association has undertaken efforts to provide legal and business resources to approximately 4,000 med spas across the United States. The aim is to collaborate with these facilities to ensure compliance with state laws and uphold safety standards.
As the FDA warns against unauthorized practices, the call for standardized regulations and increased vigilance becomes imperative to safeguard public health.

ⓒ 2025 TECHTIMES.com All rights reserved. Do not reproduce without permission.




