Pfizer and BioNTech's vaccine is aiming to debut is COVID-19 "booster shots" for approval to the US Food and Drug Administration (FDA) for inoculation among adults. The need for the booster shot is debated by different health organizations, especially for those who have received their complete shots already, with some thinking about the "mix and match" of these shots.
Pfizer, BioNTech's COVID Booster Shot for FDA Approval
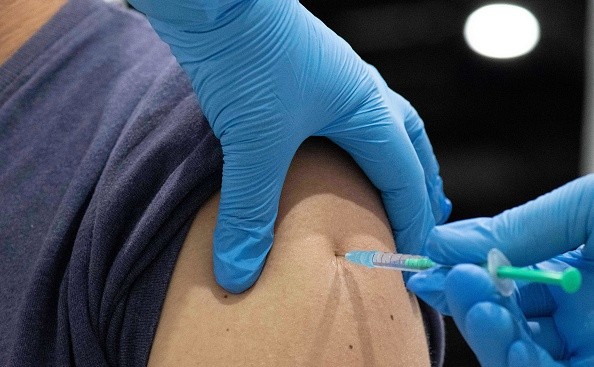
According to the report by The Washington Post, Pfizer and BioNTech are yet again approaching the FDA for its approval of their COVID-19 booster vaccine that is needed to further the immunity of a person. Several people familiar with the matter have confirmed this approval process says The Post, but none is confirmed by the FDA and Pfizer yet.
Currently, it remains unknown if the booster is effective or has done its phases of clinical trials, which are needed to ensure its safety and inoculation to different people. The vaccine shot was said to be intended for adults only, but it may be so that other booster shots would be available or eligible for the inoculation of the younger population.
Read Also : US Reopens Borders After 20-Month Entry Ban Due to COVID-19 - Travelers Must be Vaccinated
Pfizer, BioNTech's COVID-19 Booster
Pfizer and BioNTech would again be one of the firsts in the world to debut a COVID-19 booster vaccine from their laboratories, bringing the mRNA shots to further the protection it has. The concern of some now is the "mix and match" that would happen, especially for those that received different brands like Moderna, Oxford and AstraZeneca, Jansen, and more.
Pfizer's COVID-19 Vaccine and Booster
Two biopharmaceutical companies have already presented their timelines with regards to a booster shot for COVID-19 vaccines, known popularly as the third shot to further empower the body's immunity. Pfizer and BioNTech's booster was said to be available by September, and on the other hand, Moderna is aiming to deliver this by year's end.
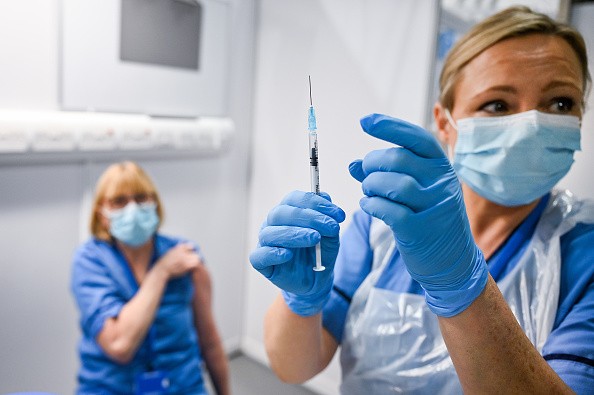
However, the World Health Organization (WHO) has slammed the need for a booster earlier this year, and the debate between agencies, manufacturers, and recipients have all meddled with its availability. The talks about a booster shot are heard again now, with Pfizer aiming for its shots to be tested and approved by the country's health agency.
Boosters are said to be important with regards to immunization among all people in the country, as it is also required for other vaccines, more commonly known for growing children to fight against diseases. The only thing that it awaits now is its approval from the FDA if the company had indeed passed its dose for approval, as well as mixing it with other vaccine doses.
This article is owned by Tech Times
Written by Isaiah Richard
ⓒ 2025 TECHTIMES.com All rights reserved. Do not reproduce without permission.




