The US Food and Drug Administration (FDA) has approved the 3D-printed Ventilator Converter part created by 3D printer manufacturer Formlab.
According to a Techcrunch report, an emergency use authorization was provided to the company that developed the 3D-printed parts which are designed to convert BiPAP machines into much-needed ventilators to effectively treat sleep apnea.
Also Read: COVID-19 Cure Update: U.S. FDA OKs Issuing Emergency Authorization to Use Injected Drug Remdesivir as Cure to Patients with Coronavirus
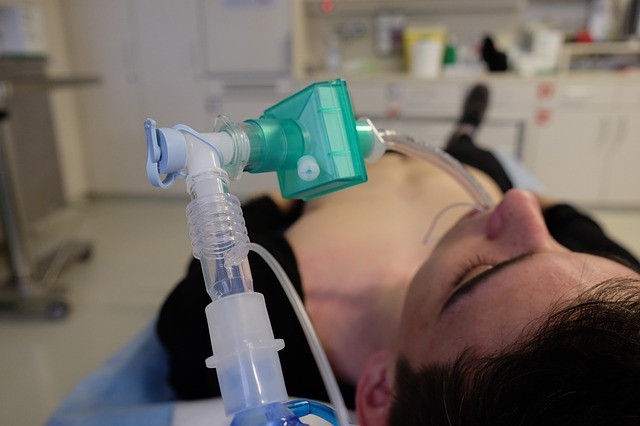
According to Formlabs, they are the first to receive an emergency use authorization from the FDA for their 3D-printed adapters. Techcrunch stated that the 3D-printer is one of the dozens of ventilators and accessories which was authorized by the FDA over the weekend.
The company is looking to provide 150 3D-printers at its Somerville, Mass plant. It also said hospitals in the US and other local governments would also have their own 3D-printers to be able to produce the ventilator convertors.
"Prior to the COVID-19 pandemic, the FDA had only authorized a handful of EUAs over a 30-year period," said Max Lobovsky, CEO of the Formlabs.
"Hospitals around the country can also use Formlabs' printers to create these adapters locally under their own practice of medicine, meaning printing the adapters at scale in the hardest-hit areas is as easy as uploading a design and pressing print," he added in the report.
FDA approves Formlabs with Emergency Use Authorization for 3D-Printed Ventilator parts: 150 to be given out in the U.S.
It was explained by Formlabs' website that the new equipment was designed to combat the shortage of ventilators and provide life-saving treatment to COVID-19 patients.
The newly developed 3D-printer adapter is designed to converter BiPAP machines into functional invasive mechanical ventilators.
BiPAP machines are usually used to treat patients suffering from sleep apnea. BiPAP machines will be converted using a small plastic T-shaped adapter which is easily produced by the hospitals' 3D printing machines across the US.
The 3D-Printers will be produced in the company's FDA-registered headquarters located in Somerville, MA.
Formlabs is currently planning to allocate 150 3D printers primarily at its headquarters to produce 3,000 parts per day once the project is fully operational. The company is also looking to distribute its product to hospitals and various government systems throughout the United States.
Formlabs is one of the 3D-printing companies which is still operating amid the COVID-19 pandemic.
They developed a swab designed for COVID-19 test kits, including other objects such as mask shields and adapters, which could convert snorkel masks into Personal Protective Equipment or PPE.
Also Read: Coronavirus Death May Happen Once Hospitals Use Chinese Ventilators, Accuse U.K Doctors
ⓒ 2025 TECHTIMES.com All rights reserved. Do not reproduce without permission.




