Antimicrobial-resistant infections have long been a looming public health threat, and the situation was exacerbated during the COVID-19 pandemic with increased antibiotic use and fewer prevention measures.
This crisis has fueled the urgency to discover new antibiotics capable of treating drug-resistant infections.
A report shared by Phys.org tells us that scientists have unveiled a promising molecule called KKL-55, which can disable a vital bacterial growth process without harming human cells.
This research, led by experts from Emory University and Pennsylvania State University, sheds light on a groundbreaking approach to tackle antibiotic-resistant bacteria.
The Global Antibiotic Resistance Challenge
According to the U.S. Centers for Disease Control and Prevention (CDC), at least 2.8 million antimicrobial-resistant infections occur annually in the United States, claiming over 35,000 lives.
The World Health Organization (WHO) predicts that, by 2050, these infections could cause up to 10 million deaths per year worldwide unless new antibiotics are developed.
The emergence of antibiotic resistance is partly attributed to the overuse of antibiotics. Bacteria continuously evolve defense mechanisms against these drugs, rendering them ineffective.
Furthermore, some antibiotics exhibit cross-toxicity, which means they can harm human cells while targeting bacteria. This presents a critical challenge that researchers are determined to overcome.
A Unique Target: Trans-Translation
To address the problem of cross-toxicity, scientists like Christine Dunham and Kenneth Keiler are focusing on inhibiting a unique bacterial mechanism called trans-translation.
This process is essential for proper bacterial ribosome function but does not have an equivalent in human cells. Dunham, a structural biologist, and Keiler, a molecular geneticist and biochemist, combined their expertise to explore this promising avenue for antibiotic development.
In human cells, the ribosome functions like a protein factory. Messenger RNA (mRNA) carries instructions from the nucleus to the ribosome to synthesize proteins. However, human cells have a quality control system to check the mRNA's integrity before translation. Bacterial cells lack this quality control, leading to issues when defective mRNA is encountered.
Trans-translation is a rescue mechanism for ribosomes stalled by defective mRNA, ensuring continued protein synthesis and bacterial cell viability. By targeting trans-translation, scientists aim to disrupt bacterial growth selectively.
Unveiling KKL-55: A Game-Changing Antibiotic
The researchers have identified a range of molecules that inhibit trans-translation in bacteria through a high-throughput screening process.
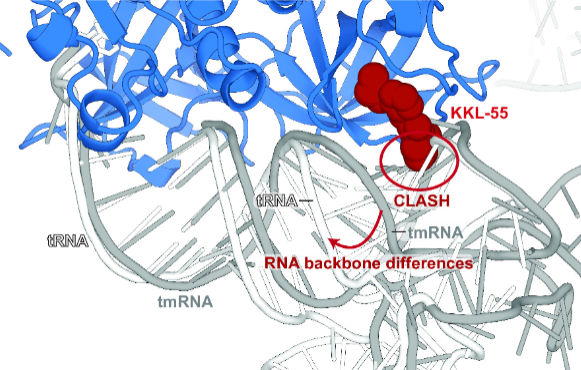
In their latest study, they honed in on KKL-55 and elucidated how it thwarts bacterial growth. Using X-ray crystallography, they captured KKL-55 in action as it interacted with a key protein, elongation factor thermo-unstable (EF-Tu).
Their findings revealed that KKL-55 effectively blocks trans-translation by binding to EF-Tu. This interaction prevents EF-Tu from binding to transfer-messenger RNA (tmRNA), a crucial component of trans-translation, thereby halting the bacterial growth process. Importantly, this specific inhibition of EF-Tu tmRNA binding presents a viable target for antibiotic development.
As scientists continue to explore dozens more trans-translation inhibitors, each represents a potential weapon to combat these relentless bacterial adversaries.
The next step is to test the efficacy of KKL-55 in treating bacterial infections in mouse models, paving the way for clinical trials and the potential development of a new generation of antibiotics.
Stay posted here at Tech Times.

ⓒ 2025 TECHTIMES.com All rights reserved. Do not reproduce without permission.




