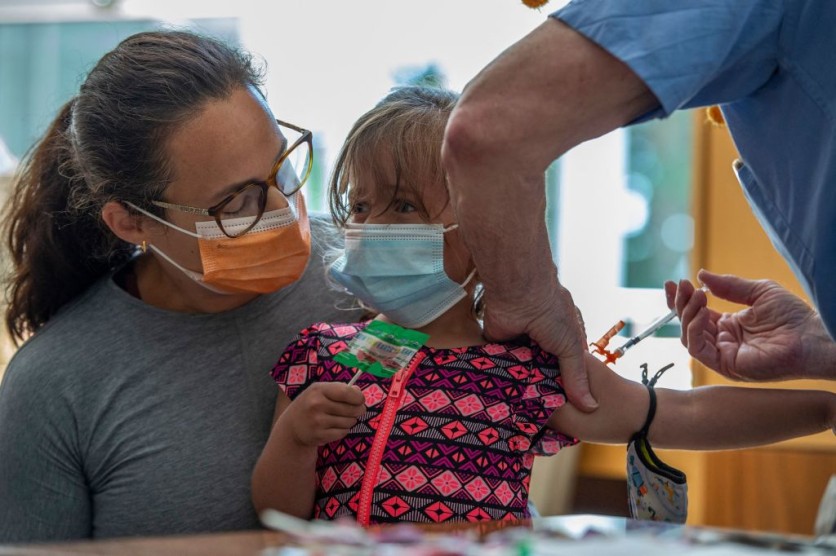
US pharmaceutical firm Pfizer requested emergency authorization for its COVID-19 booster dose for school-age children aged 5 to 11 on Monday, Sept. 26, in an effort to combat recent Covid subvariants of Omicron, according to a report by WION.
BioNTech, the German company that collaborated with Pfizer on the vaccine research, submitted a request to the US Food and Drug Administration (FDA) asking for an Emergency Use Authorization (EUA) for a dose of the vaccine weighing 10 micrograms.
Read also : Moderna Sues Pfizer, BioNTech for Use of mRNA Technology in COVID Vaccines-Pfizer Responds
BA.4 and BA.5 Subvariants
The booster dose is intended to neutralize the original Covid strain as well as the Omicron variant and its following BA.4 and BA.5 subvariants.
Studies show that roughly 90% of all new cases in the US at this time are BA.4 and BA.5 subvariants. If the FDA approves the bivalent booster, it still needs to pass a final inspection by the USA's top government health organization, the Centers for Disease Control and Prevention (CDCP).
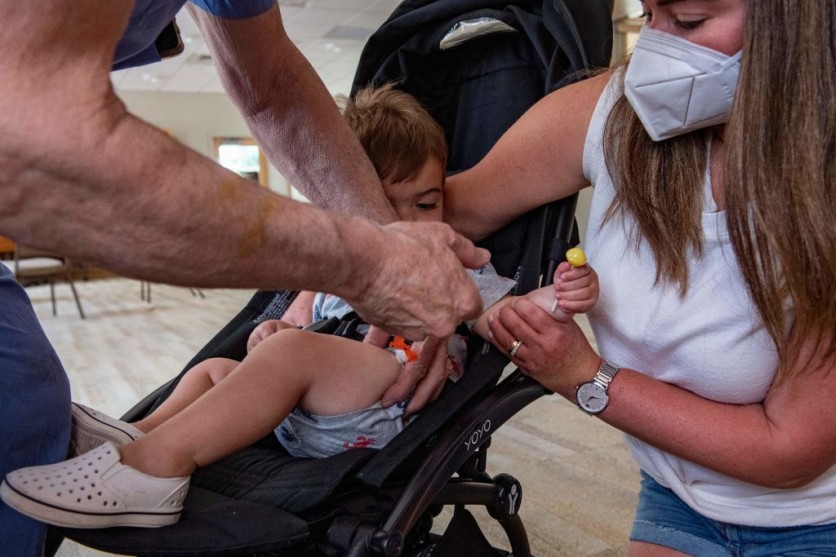
As of now, anyone 12 years of age or older can receive a 15-microgram bivalent booster dose of the vaccine thanks to approval from the US FDA.
While the number of Covid cases in the nation is declining, FDA Commissioner Robert Califf thinks that if people spend more time indoors later this fall and winter, the US may experience yet another increase in cases.
Over 616 million vaccination doses have reportedly been given across the US to date, according to CDCP data. Since June 18, 2022, more than 1.24 million kids older than five have received at least one immunization dose, while 4.4 million Americans have already received the updated booster dose.
Pfizer and BioNTech also stated that they would be applying for a similar approval for Europe soon.
"An application to extend the Omicron BA.4/BA.5-adapted bivalent vaccine marketing authorization to include children ages 5 through 11 years will be submitted to the European Medicines Agency (EMA) in the coming days," the companies wrote in a joint statement.
Related Article : Modified Pfizer Omicron-Based Vaccine Boosts Defense Against Variant? FDA To Discuss Tweaked COVID-19 Medicine
This article is owned by Tech Times
Written by Joaquin Victor Tacla

![Apple Watch Series 10 [GPS 42mm]](https://d.techtimes.com/en/full/453899/apple-watch-series-10-gps-42mm.jpg?w=184&h=103&f=9fb3c2ea2db928c663d1d2eadbcb3e52)


