Moderna's original COVID-19 vaccine shot got its FDA approval after submitting it for more than a year and after its massive distribution as an Emergency Use Approved shot. The pharmaceutical company's shot is only the second vaccine to get the FDA approval right next to Pfizer and BioNTech, which also use the same mRNA ingredients for the shot.
Moderna's COVID-19 Vaccine Gets its FDA Approval
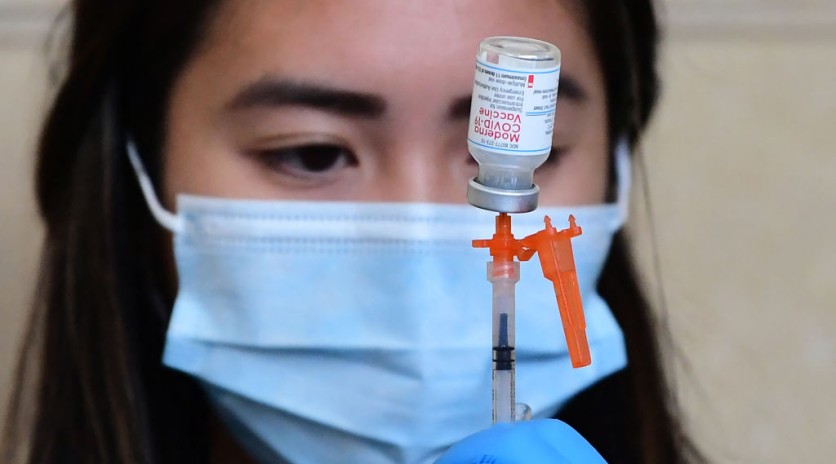
Earlier today, Moderna released a report regarding the Full FDA Approval that the pharmaceutical company received from the US Food and Drug Administration (FDA). The approval is for the COVID-19 vaccine from Moderna that uses the mRNA base for its shot, becoming only the second immunization shot approved for its use.
Other vaccines are still under the Emergency Use Authorization (EUA) provisions from the FDA, and this new approval ensures that the vaccine is well-studied and examined.
According to the FDA, this is a critical step in the fight against COVID-19, especially as it needs to ensure the regular dosage of the shot under the name "Spikevax," available for 18 years and older.
Moderna: Second to Pfizer, mRNA COVID Vaccine
Moderna is second to Pfizer's approval in the US FDA for the COVID Vaccine, and that is a lot for the company as it is also the other pharmaceutical that used mRNA. It is important to note that Pfizer and Moderna took a risk with the mRNA base as it is an experimental ingredient for vaccines before COVID. It was only during the pandemic that the makers could apply it.
Moderna and its Focus on mRNA Vaccines
Moderna is one of the two companies that focused on creating vaccines using the experimental mRNA shots, and it gave them success with making one for COVID-19. The shots that have the mRNA ingredients on them produce the highest rate of protection from all vaccines present now, even in booster shots for people, which is the focus now.
However, while Moderna trails Pfizer for the COVID shot, the Cambridge-based pharmaceutical company also aims to develop new vaccines using the ingredient. One of the many focuses of Moderna is HIV, and the vaccine will be a first in the industry, focusing on the immunization against the virus itself and the use of mRNA for shots outside COVID.
The company's focus on mRNA vaccines is a massive contribution to the world as it is a rapidly developing industry where Moderna thrives and specializes.
However, it may not be the "silver bullet" against the virus or infection. However, it still holds a promising vaccine that features high levels of protection, as what the FDA-approved COVID shot from Moderna brought.
Related Article : Moderna's COVID-19 Booster to Produce 2nd Shot, But Experts Doubt its Need Now; Shorter Waiting Time for Jab
This article is owned by Tech Times
Written by Isaiah Richard
![Apple Watch Series 10 [GPS 42mm]](https://d.techtimes.com/en/full/453899/apple-watch-series-10-gps-42mm.jpg?w=184&h=103&f=9fb3c2ea2db928c663d1d2eadbcb3e52)



