The Johnson & Johnson COVID-19 vaccine was recently criticized after a serious but rare side effect was discovered. This health issue is specifically the blood clots, which already lead to some death cases.
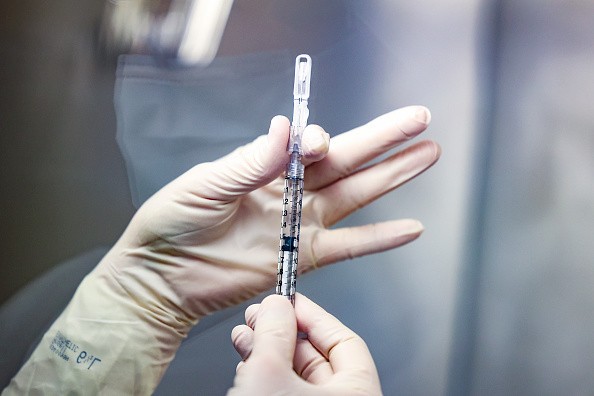
Now, Johnson & Johnson and AztraZenaca developers claimed that if they modify their COVID-19 vaccines, the risk of blood clots could be reduced. As of the moment, both health tech firms are now conducting early-stage research into vaccine modifications.
This activity will help them identify the efficiency of the method when it comes to reducing or even eliminating the risk of blood clots. Once that happens, it will not only make people safe, but it could also encourage more people to get vaccinated, especially since strains are now appearing, such as the new COVID-19 Delta variant.
As of the moment, medical experts claim that blood clotting, also known as vaccine-induced immune thrombotic thrombocytopenia or VITT, is linked to both vaccine shots. On the other hand, they were also able to find how these COVID-19 vaccines lead to blood clots.
Johnson & Johson, AstraZeneca COVID-19 Vaccines To Be Modified?
According to CNBC's latest report, the latest blood clotting findings, which explain how side effect forms, lead many medical researchers to believe that vaccine modification could help reduce the risk of the so-called blood clotting.

Also Read : CDC, Fauci Thinks COVID-19 Boosters Are Not Essential As of The Moment, to Address its Need in Briefing
This information was confirmed by an anonymous vaccine developer. However, it was not disclosed if he is working for Johnson & Johnson or AstraZeneca.
"Johnson & Johnson remains committed to helping end this deadly pandemic as quickly as possible, and we continue to believe in the positive benefit-risk profile of our vaccine," said Johnson & Johnson.
"We're actively working with the regulators and scientific community to understand these extremely rare blood-clotting events, including information to drive early diagnosis and intervention, and appropriate treatment," added AstraZeneca.
On the other hand, FCC released a statement, saying that the blood clotting side effect is rare. But, the Food and Drug Administration also warned Johnson & Johnson COVID-19 vaccine that it is linked to another serious side effect called Guillain-Barre syndrome, which is an autoimmune disorder.
Guillain-Barre Syndrome Outweighs J&J COVID-19 Vaccine's Efficiency?
The Verge reported that FCC believes that the new serious but rare Guillain-Barre Syndrome still outweighs Johnson & Johnson COVID-19 vaccine's efficiency.
The health agency added that the COVID-19 vaccine's new side effect already affected more than 100 individuals. On the other hand, CDC is also now concerned with the new health issue that Johnson & Johnson COVID-19 vaccine poses.
For more news updates about Johnson & Johnson and other COVID-19 vaccines, always keep your tabs open here at TechTimes.
Related Article : COVID-19: Old Belgian Woman Who Died in March Had Two COVID-19 Variants In Her Body [REPORT]
This article is owned by TechTimes
Written by: Griffin Davis
![Apple Watch Series 10 [GPS 42mm]](https://d.techtimes.com/en/full/453899/apple-watch-series-10-gps-42mm.jpg?w=184&h=103&f=9fb3c2ea2db928c663d1d2eadbcb3e52)



