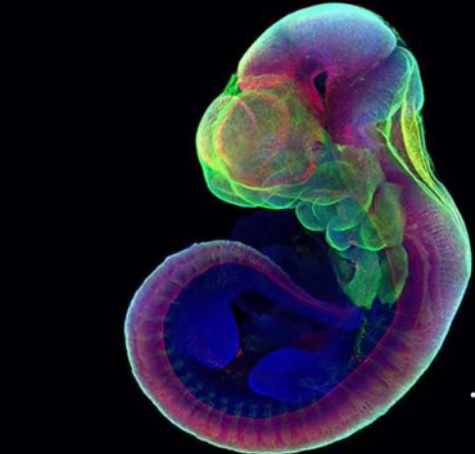
Scientists were able to grow the embryo of a mouse outside of the womb for the first time in tech history. A group of researchers from the Weizmann Institute of Science in Israel said that they have successfully grown more than 1,000 mice embryos for six days using a mechanical device process.
Mice embryo is grown in a mechanical device
The study was published on Mar. 17 in Nature and the first part of the experiment saw that the team remove the mice from their mothers' wombs after five days.
Dr. Jacob Hanna told The New York Times, one of the researchers on the project, said that his team has since managed to take an embryo from a female mouse right after fertilization and grow it for 11 days. The lab-grown embryos are identical to their real counterparts.
The team then spent seven years making the machine that has helped them with their research. It is a two-part system that consists of an incubator and a ventilation system.
Each of the embryos floats in a vial that is filled with a special nutrient-laden fluid. A wheel gently spins the mice so that they do not get attached to the wall of their temporary home.
The wheel prevents the embryos from deforming and dying while inside the mechanical womb. The attached ventilator also gives the mice oxygen while maintaining their environment's flow and pressure.
According to the study, it takes 20 days for a mouse to gestate to the point where it can survive outside of the womb. So far, the mechanical womb that Dr. Hanna and his team of researchers have created can help sustain the mice through 11 days of growth.
The embryos need blood supply and not just nutrients, and that is the next challenge that the team of scientists plans to solve. , one potential solution that was presented is including an artificial blood supply that could connect the placentas of the mice.
Are humans next?
Dr. Hanna's team did not create the mechanical device to disrupt the natural order. They are using their process to study how factors like environmental conditions and genetic mutations can affect a fetus's growth while inside the womb.
So the answer is no, scientists are not planning to use the mechanical womb to nature human embryos and help develop a human child.
Until this study, scientists had turned to frogs and worms, both non-mammals, to study organs and tissues' development. The study, which UCSB, published, proves that animals such as frogs and worms have similar tissues and organs.
The findings have important implications for medicine, including cancer, the study of birth defects, and tissue engineering.
Joel Rothman, the associate professor of molecular biology at the University of California, Santa Barbara, said that the studies show that animals that are very distantly related show something in common that had not been expected.
Related Article : Trials on Growing Babies in Ziplock Bags Show Promising Results
This article is owned by Tech Times
Written by Sieeka Khan
ⓒ 2025 TECHTIMES.com All rights reserved. Do not reproduce without permission.




