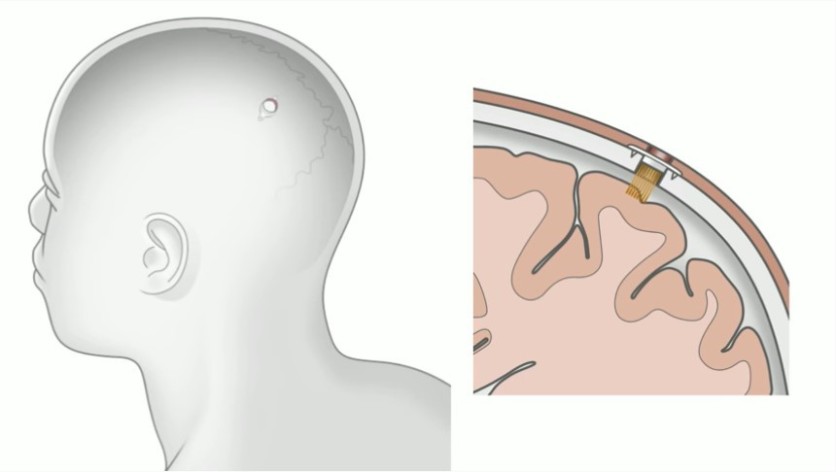
On Friday, Aug. 28, Elon Musk will officially released the demo video of its much-awaited yet controversial Neuralink brain chip. Hours from now; we will see what this brain chip would look like, what it can do, and if all of Elon's claims were true about this chip.
But here's something that the billionaire may have forgotten about his device: it still has a pending approval from the Food and Drug Administration (FDA).
Elon Musk's Neuralink coming soon!

A lot of things are already known about Neuralink's brain chip. But one thing that has been missing out from this device is the most important thing: the FDA approval.
In 2019, report says that the San Francisco-based company had submitted its FDA approval request sent to the agency. At the time, Musk confidently said that the permit will surely be accepted-- but admits it requires "a long time."
"It's not going to be like suddenly Neuralink will have this incredible new interface and take over people's brains," said Musk. "It will take a long time, and you'll see it coming. Getting FDA approval for implantable devices of any kind is quite difficult and this will be a slow process."
FDA approval is one of the most important factors to identify whether an experiment is safe to be used by a population of people.
Moving forward hours from the launch, there are still no update about the FDA approval of the controversial brain chip. Meaning to say, that there are still no permit to begin with.
Neuralink claims that human trials for the device will be set next year. To make sure that everyone woud be safe to use it, a new version of the brain chip will be created to be "only intended for patients with serious unmet medical diseases."
Its impossible for Neuralink to get the permit
Though FDA already received the request for approval, experts conclude that Elon may have difficulties to convience FDA to sign it.
Bloomberg reported that Neuralink uses a material that is not approved by the federal agency called flexible polymers. This type of material does not last a decade in the human body. Which violates the minimum timeframe set by the agency.
"If you want to test whether something can last 10 years, you really have to wait 10 years," says Matt Angle, chief executive of Paradromics Inc., an Austin, Texas-based brain-machine interface company.
On the other hand, Neuralink may seemed to be doing something else to finish its approval. According to two anonymous former employees, the company had explored the possibility of pursuing human trials in China or Russia in order to successfully get the approval.
Of course, nothing's sure for now. Let's wait hours from now.
UPDATE: Elon Musk announced during the Neutralink Livestream Demo on Aug 28 that the company has already secured FDA Approval in yet another breakthrough. Read here.
This article is owned by Tech Times
Written by Jamie Pancho

![Apple Watch Series 10 [GPS 42mm]](https://d.techtimes.com/en/full/453899/apple-watch-series-10-gps-42mm.jpg?w=184&h=103&f=9fb3c2ea2db928c663d1d2eadbcb3e52)


