Massachusetts Institute of Technology (MIT) engineers have created a reusable face mask, which they claim to have the same efficacy as the N95 masks.
The MIT engineers worked with Brigham and Women's Hospital to design the Injection Molded Autoclavable, Scalable, Conformable (iMASC). It can be used multiple times after simple sterilization.
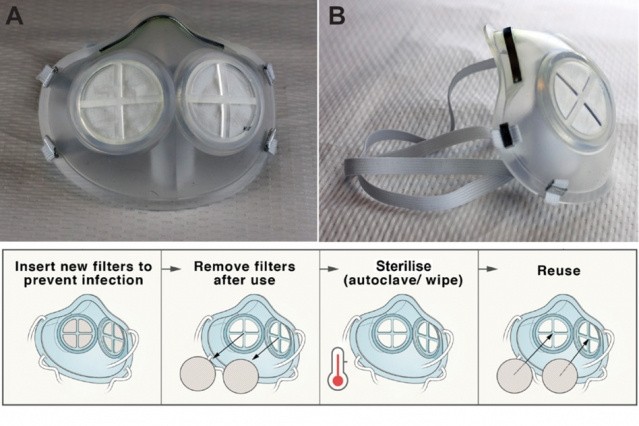
Like the N95, iMASC is made of silicone rubber and uses polypropylene fibers, which are specially designed to filter out tiny viral particles. It only uses two round replaceable filters in the front of the mask, so less material is required.
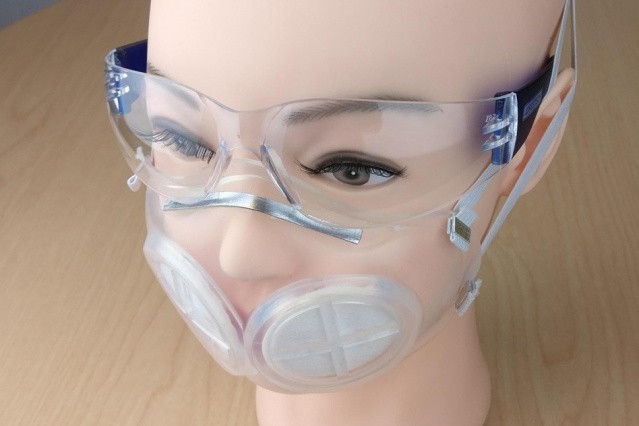
Moreover, research engineer Adam Wentworth wrote in a press release that the iMASC uses a lot less material compared to an N95 mask. "The filters can be popped in and then thrown away after use," he said
With heightened demand for N95 respirators during the coronavirus pandemic, the supply of medical-grade face masks has always been a concern, particularly as some states and countries have now made wearing masks mandatory. Unfortunately, some health workers are forced to wear single-use masks for days due to lack of supply.
"One of the key things we recognized early on was that in order to help meet the demand," said MIT Mechanical Engineering Assistant Professor Giovanni Traverso who is also the lead author of the research. He also said they want to restrict themselves to methods to scale down the iMASC while maximizing its reusability.
Moreover, the researchers wanted to produce a mask that can be sanitized tried various ways, so they tried cleaning the iMASC by autoclaving as well as soaking it in a bleach solution and isopropyl alcohol.
The iMASC research was funded by the MIT Department of Mechanical Engineering, Brigham and Women's Hospital, the National Institutes of Health, Gilead Sciences, Philips Biosensing, E-Ink Corporation as well as the Prostate Cancer Foundation and Hans and Mavis Lopater Psychosocial Foundation.
iMASC passed the test
To check if the iMASC works, 20 health professionals were put in a hood where an aerosolized sugary solution has been sprayed from time to time. The subjects were asked to perform some breathing exercises and head movements.
Throughout the test, none of the subjects have tasted the solution while nearly all of them rated the breathability of the masks as either excellent or good. Also, 60% of the subjects said they are willing to trade the wearing of iMASC to an N95 or surgical mask.
One of the lead authors James Byrne, a radiation oncologist at Brigham and Women's Hospital, said that there will always be a need for masks, either in the health care setting or in public. "We know that COVID is not going away until a vaccine is prevalent," he added.
The iMASC will still undergo further testing and approval from the Food and Drug Administration as well as the National Institute for Occupational Safety and Health before it can reach the market.
Meanwhile, the MIT engineers are currently working on improving the iMASC based on feedback from healthcare workers. They are also looking for a partner company to support its production once it is approved.
Read also: Your COVID-19 Facemask Might Come From China's Controversial Labor: What is Uighur Labor?
ⓒ 2025 TECHTIMES.com All rights reserved. Do not reproduce without permission.




