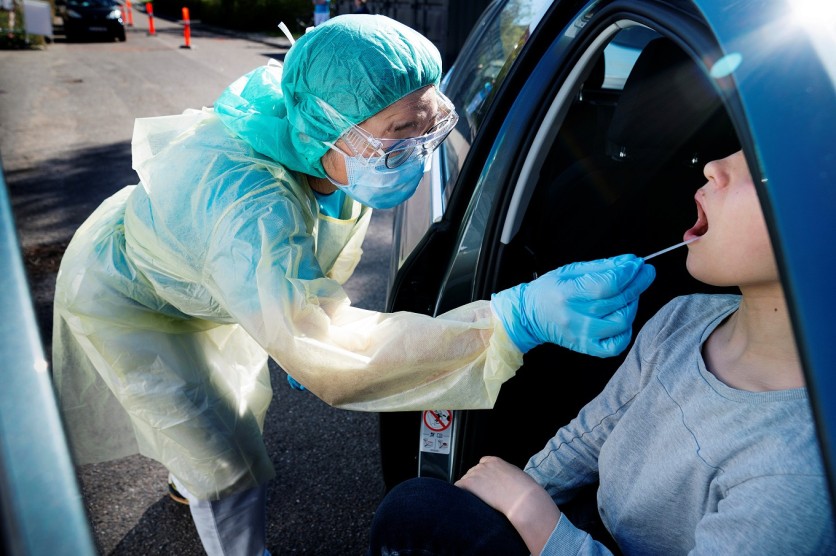
Testing is an essential part of flattening the curve of COVID-19 cases. Also, this is a way to figure out how to stop the spread. Once everyone is tested, it is possible to isolate the ones with COVID-19, especially those who are asymptomatic--and this might be achieved with rapid testing kits a recently invented one.
New Rapid COVID-19 Test
According to a report by the Daily Mail, this rapid coronavirus test is made by a team of experts from the Michigan State University's College of Osteopathic Medicine, which could produce results in as fast as five to seven minutes.
Typically, coronavirus test kits today can produce results from three to four days.
The test kit is developed by Brett Etchebarne, an assistant professor at the university and a medical physician.
Etchebarne created the COVID-19 rapid test to help emergency doctors and nurses test as many patients as possible and provide them with swift results, which could help the experts make decisions quickly.
Analyzing More Samples at Once
What makes the test even more helpful is that it could analyze up to 22 samples in one go, making it faster to test the public and quickly point out who needs to be isolated and monitored.
Additionally, it does not require the invasive nasal swab that is commonly needed with past tests that makes the procedure uncomfortable for the patients, especially to kids and the elderly. Instead, the samples are acquired from the mouth.
Based on the Lansing State Journal, the coronavirus test can be completed with the use of a real-time polymerase chain reaction.
There is another way to test swabs from suspected COVID-19 patients, but it takes 30 minutes to show results. It works by heating the sample and using a color change process, which could detect the SARS-CoV-2 virus even at low levels.
"I used what the CDC provided and made up my own primer sets, which are fragments of the target DNA or RNA that you can use to amplify a region of that genetic element," the researcher said.
When Will it be Available?
Although the rapid coronavirus test is promising, it still needs to be approved by the Food and Drug Administration (FDA) before the public can use it.
To acquire their approval, the researchers need to go through many different regulatory hoops, but they are hopeful that it will get the "go" signal from the FDA in a few weeks.
As of now, the team is working on getting the coronavirus test validated by the Clinical Laboratory Improvement Amendments lab, but Etchebarne said his team already knows what to do since this isn't the first time they created a testing kit.
He is also developing a rapid respiratory panel that detects illnesses like the flu and pneumonia.
"To me, it's a logical next step to develop the technology in real-time for real-time infections," the researcher said.
According to MSU's College of Osteopathic Medicine Dean, Andrea Amalfitano, Etchebarne's test, if approved by the FDA, will be part of a broader strategy against COVID-19 that may include finding antibodies from people who were infected and might have formed an immunity to the virus.
ⓒ 2025 TECHTIMES.com All rights reserved. Do not reproduce without permission.




