The Food and Drug Authority (FDA) launched a website to gather plasma donations from COVID-19 survivors and aid the creation of treatment for coronavirus.
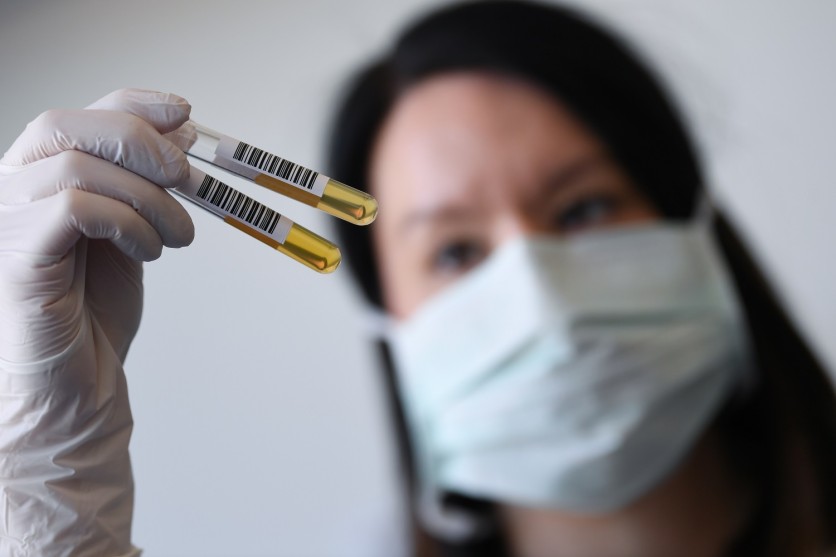
The FDA is calling for donations of convalescent plasma, which is one of the measures being pursued to develop an effective treatment for COVID-19.
This means extracting the liquid component of blood from people who have already fully recovered from COVID-19 and use it to cure the disease. These survivors develop antibodies or protein components that could help finally treat the infection caused by the SARS-CoV-2 virus.
Currently, there is still no approved treatment for coronavirus, which has now recorded at least 2,166,832 cases worldwide and 144,515 deaths. Meanwhile, the FDA is seeking the help of 546,269 patients who have recovered after contracting the disease.
It has created a dedicated website for COVID-19 plasma donations. The website provides pertinent details about the program and how survivors could help.
The new FDA website also lists the requirements for donors. Primarily, this calls those who have fully recovered from the disease for at least 14 days to consider donating plasma to help save other patients' lives. Interested individuals must bring a negative laboratory test for COVID-19 disease to any nearby local blood center or the American Red Cross and proceed with the donations.
The survivor's plasma: how it helps?
Using convalescent plasma for treatment is not a new concept. It has been used already since the 1918 Spanish flu pandemic, although it brought various results. The Ebola virus treatment attests to the success of convalescent plasma use.
Doctors will inject COVID-19 survivors plasma to infected patients. Ideally, it boosts the latter's immune system and adapts to the disease, leading to passive immunity.
The advanced science and technology could improve the efficacy of recovered plasma as a treatment method. The recent tests made in China give positive results on the process.
On Mar. 27, researchers from China reported the recovery of five patients who were treated for severe COVID-19 infections using the convalescent serum from survivors. Although all patients were on ventilators, viral titers dramatically dropped to zero 12 days after receiving the serum.
However, patients also received interferon and lopinavir/ritonavir before and during the treatment, so the role of antivirals is still unclear. Recently, experts have excluded displopinavir-ritonavir combination as COVID-19 treatments.
Various studies test the safety and effectiveness of convalescent plasma as a COVID-19 treatment. These include clinical trials and single-patient treatment authorizations for emergency investigational new drug approvals from the FDA.
With the increasing researches, there is a growing need for plasma supplies. With the creation of the new website, FDA hopes to encourage more donations.
On Apr. 13, the FDA has provided a guide for these researches to ensure the process agrees with existing protocols. The FDA recommendations include:
- pathways for the use of investigational COVID-19 convalescent plasma
- patient eligibility
- collection of COVID-19 convalescent plasma, including donor eligibility and donor qualifications
- labeling
- record-keeping
While the FDA does not collect the plasma, it directs the possible donors to FDA-registered blood facilities.
![Apple Watch Series 10 [GPS 42mm]](https://d.techtimes.com/en/full/453899/apple-watch-series-10-gps-42mm.jpg?w=184&h=103&f=9fb3c2ea2db928c663d1d2eadbcb3e52)



