The United States Food and Drug Administration (FDA) gave an emergency approval on using blood as part of the Coronavirus testing process as announced on Wednesday last week. This means that human blood is now one of the factors to be analyzed to determine whether a recovered Coronavirus patient is already immune to the virus or not.
FDA approves use of blood for Coronavirus testing
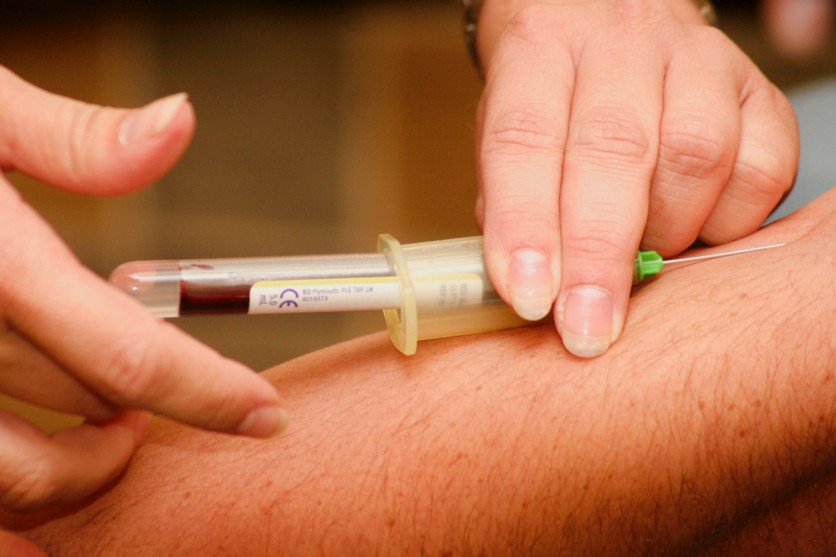
As first reported in Daily Mail UK, this will be the first blood test approved by the FDA to use against the Coronavirus. A serology test, which is the term used to describe the blood test, was promoted by manufacturer Cellex Inc., a medical device company originally based in North Carolina.
"Based on the totality of scientific evidence available to FDA, it is reasonable to believe that your product may be effective in diagnosing COVID-19," FDA chief scientist Denise Hinton wrote in a letter to James Li, the CEO of Cellex. "The known and potential benefits of your product when used for diagnosing COVID-19, outweigh the known and potential risks of your product."
Serology test to use whether a recovered patient is COVID-19-free or not

The FDA said that there are antibodies found in the blood of a recovered patient that are "generally detectable ... several days after the initial infection." This means that if authorities test the blood of newly-recovered victims, they will be more accurate to certify them as COVID-19-free. They can then allow these patients to go outside and be with their families after recovery.
It will also be an important strategy to allow them to work outside of their homes to be able to jumpstart the economy while others are in a lockdown.
How does the test work?
Compared to the standard Coronavirus testing where doctors use the process of swab testing a patient's throat or nose, the serology test will require blood from the patient's veins. Just like swab testing, a certified laboratory will identify the genetic material and determine if someone is still infected with the virus or not.
As explained by the FDA, the test could help provide data on just how immense the impact of Coronavirus has been around the world. The test does not just identify who is infected or not; its main purpose is to determine whether a newly-recovered Coronavirus patient is really free from COVID-19 or not.
As of April 6, the death rate in the US has increased to over 9,000. Meanwhile, there are now more than 300,000 confirmed COVID-19 cases, and 17,000 recoveries since day one of the infection.
![Apple Watch Series 10 [GPS 42mm]](https://d.techtimes.com/en/full/453899/apple-watch-series-10-gps-42mm.jpg?w=184&h=103&f=9fb3c2ea2db928c663d1d2eadbcb3e52)



