The growing ventilator shortage for COVID-19 patients has become one of the main issues faced by frontline medical workers during the current pandemic. Because of this, there has been a need for uncommon solutions from both the private and public sector to help address medical equipment deficit. And some organizations and individuals have responded with their recommended alternatives.
The FDA has been improving its policies and restrictions to respond and adapt to the ongoing medical equipment shortage in the face of the Coronavirus epidemic. A newly developed ventilator called Sleep Apnea Retrofit was designed to help resolve this equipment deficit. Will this be approved by the FDA in time? Will it even pass the FDA's standards for medical equipment?
COVID-19 Ventilator Rapid Response Team newly designed ventilator
According to a Techcrunch report, the FDA is now working with a group of medical experts, engineers, and researchers from UCSF and UC Berkeley to develop a solution for the ongoing shortage of medical equipment needed to help coronavirus patients.
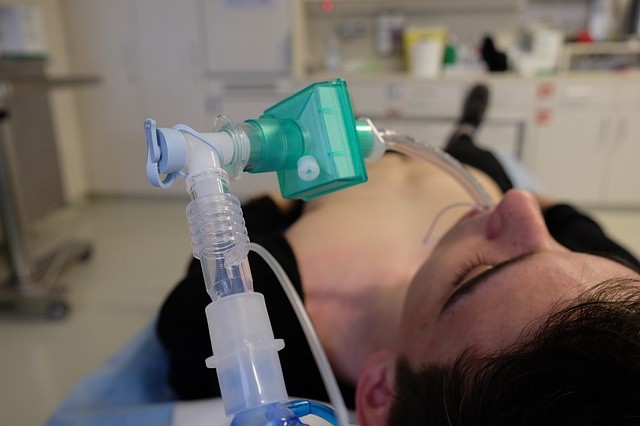
COVID-19 Ventilator Rapid Response Team, a group of doctors, pulmonary care physicians, medical and engineering professors, has been working to address the shortage by modifying their existing Continuous Positive Airway Pressure machines or CPAP which are usually used for treating people with sleep apnea.
Now, they are needed to keep COVID-19 patients breathing in the ICU, acting as ventilators for intubation. The newly designed ventilator is called Sleep Apnea Retrofit.
Sleep Apnea Retrofit will address the medical shortages
CPAP machines or Sleep Apnea machines maintain oxygen levels and prevent unwanted rousing and snoring when asleep, ensuring the patient's airway doesn't get blocked while they are sleeping. The problem these machines have is that they are not designed to be used continuously by patients who have severe conditions and can't breathe on their own.
The group of doctors known as COVID-19 Ventilator Rapid Response Team has figured out a way to modify CPAP machines for continuous and longer use for coronavirus patients with severe symptoms. The group behind this has adapted the CPAP machine's hardware using a tube that can be used for intubation.
This medical improvement was led by Dr. Ajay Dharia, a critical care physician and engineer from UC Berkeley, who focuses on pulmonary issues in the ICUs at 3 Bay Area hospitals.
The FDA has already issued a guidance that orders medical professionals and health care facilities when using breathing devices that were not originally designed as ventilators. Using this, the COVID-19 Ventilator Rapid Response Team came up with the idea to modify the existing machine and they devised a different approach to meet the FDA's medical guidlines. The group is now seeking FDA approval because they want to start producing the modified Sleep Apnea Machines in large quantities.
To further help their cause, the group is also enlisting donations of CPAP or Sleep Apnea Machines that are not currently being by individuals and organizations on their website. The COVID-19 Ventilator Rapid Response Team is confident that their newly designed CPAP machines will be approved and can help the patients who are severely affected by the coronavirus.
Also Read: USA Not Part of WHO's Team of Coronavirus Cure Hunters; But Why?
ⓒ 2025 TECHTIMES.com All rights reserved. Do not reproduce without permission.




