After receiving scrutiny over its ID NOW rapid test earlier this month, Abbott Laboratories has bounced back with an analysis of data from an on-going study that shows their kit is highly accurate to test for the coronavirus.
Abbott's data has shown that the test has nearly 95% accuracy in detecting positive cases while producing results in minutes, compared with two other laboratory tests. These results contradict other studies that question the test's accuracy.
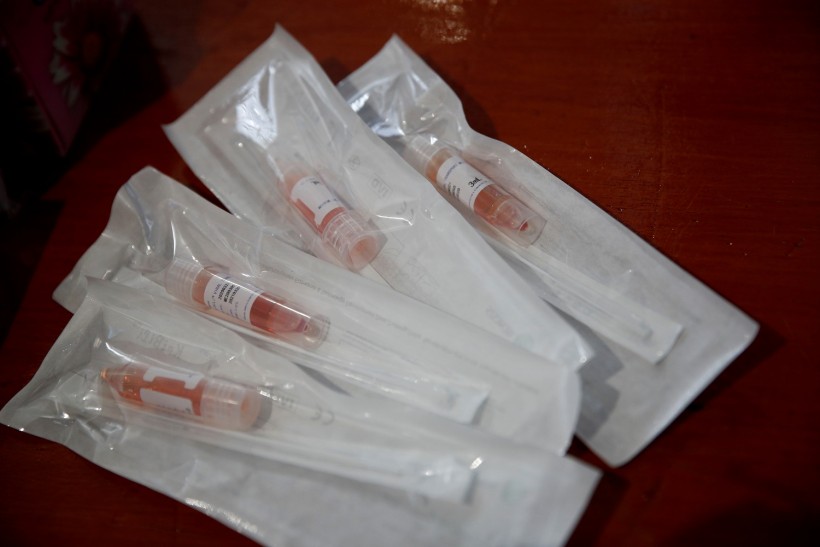
Nasal swab test kits are pictured at a new medical facility set up for testing migrant workers residing in dormitories for the coronavirus disease (COVID-19), in Singapore May 10, 2020.
The ongoing study has analyzed samples from 256 patients and then compared the results to a test created by the US Centers for Disease Control. According to the partial data from the said study, the ID NOW Rapid test showed that it detected the virus with 94.7% accuracy while giving 98.6% correct negative results, according to Bloomberg.
The pharmaceutical company said that data from its study and two others suggest that ID NOW rapid test works best on patients who are tested after the onset of the illness' symptoms.
Last week, the US Food and Drug Administration (FDA) warned that Abbott's speedy coronavirus diagnostic test could be inaccurate and give false-negative results. The FDA also said that early data suggested that it may sometimes fail to detect the illness.
At the same time, researchers from New York University said that the ID NOW test could be missing from a third to nearly half of positive cases. This test, however, was not yet peer-reviewed.
In contrast, Abbott was quick to defend itself saying that the NYU study involved a small number of patients, and the ID NOW test was not used according to how it was intended to be which may have led to the low-efficiency rate.
Meanwhile, the FDA said it will continue to review data on the test kit's accuracy while Abbott agreed to conduct studies on its product. Each of the studies will include at least 150 COVID-19-positive patients in various healthcare settings.
Abbot's ID NOW coronavirus rapid test kit shows promising results after last week's scrutiny
The ID NOW coronavirus rapid test kit was developed by Abbott Laboratories and promised to deliver results within 5 to 13 minutes.
According to a report by Tech Times, Abbott has already shipped more than 1.8 million test kits since the FDA issued an emergency use authorization to fast-track its approval three days after President Donald Trump called it a game-changer during a White House event.
Last week, the FDA announced that patients who received negative results using the ID NOW rapid test may need to go through a more sensitive test to confirm the results. The agency also said they will continue to study available data while working with Abbott to have additional methods to analyze the results.
The FDA also vowed to be transparent with any information, while Abbott promised to send letters advising those who received negative test results to undergo a confirmatory test.
The pharmaceutical company's shares fell 1.8% to $92.16 in New York last week after it started up 6.1% early this year.
Read also: Drug Trafficker Sentenced to Death via Zoom Call During Coronavirus Lockdown